US FDA Approves Elon Musk’s Neuralink for Human Trials
- Kingston Bailey
- Science
- Technology
- Trending
- May 31, 2023
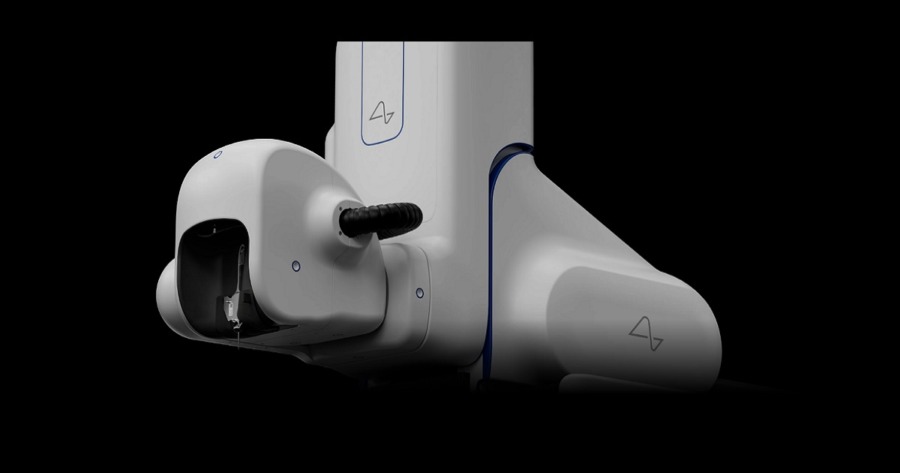
Potential for Groundbreaking Neurological Advancements Amid Controversy
In a significant development, the US Food and Drug Administration (FDA) has granted approval for Elon Musk’s Neuralink company to conduct human trials. This approval marks a pivotal moment for the Neuralink project, which aims to develop advanced brain-machine interface technology to address neurological disorders and enhance human capabilities. However, the road to FDA approval has not been without hurdles.
Neuralink, founded by Musk in 2017, focused on developing brain-machine interfaces (BMIs) that establish a direct link between the human brain and external devices. The goal is to create a seamless connection that can enhance cognitive abilities, treat neurological disorders, and potentially revolutionize human-computer interaction.
Elon Musk’s Neuralink had previously applied for FDA approval in 2022 but was initially denied due to concerns over the safety and efficacy of the technology. The FDA’s role is crucial in ensuring that new medical interventions are thoroughly evaluated and meet stringent regulatory standards to protect patient safety.
However, the recent approval for human trials signifies a significant step forward for Neuralink. It suggests that the FDA has deemed the technology ready for further exploration and human testing, indicating growing confidence in its potential benefits.
Neuralink has also faced scrutiny from the Department of Transportation due to allegations of improper disposal of brain tissues from implanted chips in monkeys. The ongoing investigation focuses on whether proper biomedical waste disposal processes were followed. It is essential to address these concerns to ensure ethical practices and maintain public trust.
Additionally, several current and former Neuralink employees have publicly expressed concerns about Elon Musk’s alleged rush to bring the technology to market. These employees argue that the development process needs to be better managed, leading to suboptimal results and compromising safety measures. Such criticism highlights the importance of proper research, testing, and transparency in groundbreaking projects like Neuralink.
Then, of course, various animal rights groups have filed complaints against Elon Musk and Neuralink, alleging mistreatment and torture of animals during the development process. These groups assert that the treatment of animals during experimentation should follow rigorous ethical standards and prioritize the well-being of the subjects involved.
Significance and Potential of Neuralink Technology
If successful, Neuralink’s brain-machine interface technology has the potential to revolutionize the field of neuroscience and healthcare. Here are a few key areas where Neuralink could make a significant impact:
1. Treatment of Neurological Disorders
Neuralink’s technology holds promise for individuals suffering from neurological disorders such as paralysis, Parkinson’s disease, epilepsy, and even mental health conditions. By establishing a direct connection between the brain and external devices, Neuralink could offer new ways to manage and treat these conditions, potentially improving the quality of life for millions.
2. Augmenting Human Capabilities
Beyond medical applications, Neuralink envisions enhancing human capabilities by integrating advanced AI and machine learning algorithms with the brain. This could lead to unprecedented advancements in memory augmentation, cognitive abilities, and communication interfaces, allowing for more efficient human-machine interactions.
3. Unlocking New Research Frontiers
Neuralink’s technology can open up new frontiers in neuroscience research. By providing unprecedented access to neural activity, researchers could gain deeper insights into brain functions, leading to a better understanding of neurological processes, consciousness, and human cognition.
Over the past year, Musk has been vocal about his concerns regarding the rapid advancement of artificial intelligence (AI) and its potential risks. However, it is notable that Neuralink, as one of the most advanced AI companies, operates at the forefront of AI-driven research. This contradiction raises questions about Musk’s stance and the need for consistent ethical practices and responsible development across all AI-related endeavours.
The recent FDA approval for Neuralink to conduct human trials signifies a significant milestone for the project. While controversies and concerns surrounding the technology should not be dismissed lightly, it is crucial to recognize the potentially transformative impact of Neuralink’s brain-machine interface technology. It could pave the way for groundbreaking advancements in healthcare, human potential, and our understanding of the human brain if successfully developed and implemented.
However, it is equally important to address ethical considerations, ensure employee well-being, and maintain transparency throughout the development process to uphold the trust of the public and the scientific community.
Image source Neuralink








