UK Approves Clinical Trials To Use Plasma To Treat COVID-19
- TDS News
- COVID-19
- United Kingdom
- April 25, 2020
UK Approves Clinical Trials To Use Plasma To Treat COVID-19. Up To 10,000 Units Of Plasma Can Be Delivered Weekly
If effective, a national programme will deliver up to 10,000 units of plasma a week to the NHS to help treat coronavirus (COVID-19) patients.
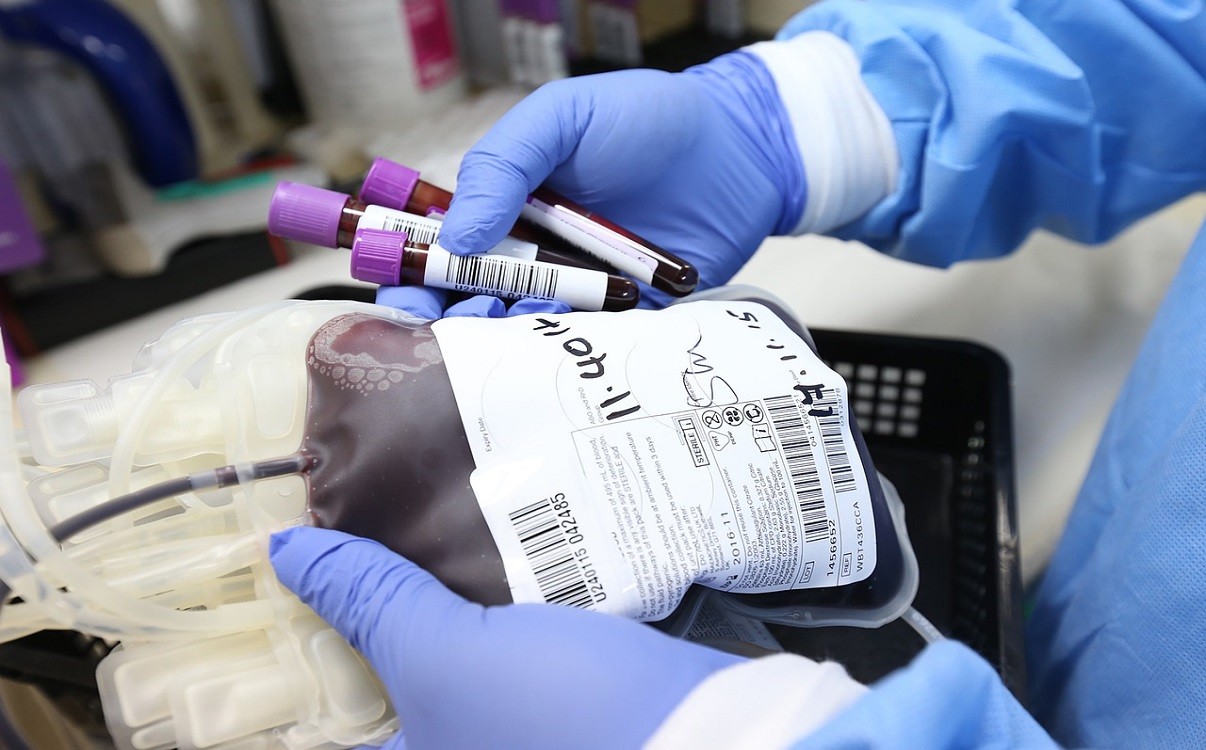
A clinical trial has been given approval to determine if plasma donated by patients who have recovered from COVID-19 can help those battling the illness. If effective, a scaled-up national programme will deliver up to 10,000 units of convalescent plasma per week to the NHS. This would provide enough convalescent plasma to treat 5,000 patients each week.
Up to 5,000 severely ill patients with COVID-19 could soon be treated each week with plasma from those who have recovered from the illness as part of a new approach to treating the virus.
The national randomised clinical trial will help to determine if plasma collected from donors who have recovered from COVID-19, known as ‘convalescent plasma’, is an effective treatment for patients who are severely unwell with the illness. Plasma from recovered COVID-19 patients can be transfused to patients who are struggling to produce their own antibodies against the virus.
In parallel with the trial, the government is scaling up the national programme for collecting plasma so the treatment can be widely rolled out if it is shown to be effective. The collection of plasma would be ramped up over April and May to deliver up to 10,000 units of plasma to the NHS every week, enough to treat 5,000 COVID-19 patients per week.
“As well as continuing to collect enough blood throughout this outbreak, we are also heavily involved in the national research response including major trials of this potential treatment.
We are rapidly building our capability to collect plasma so that we can quickly move into supplying hospitals at scale, should the proposed trial demonstrate patient benefit.” Dr Gail Miflin, Chief Medical Officer, NHS Blood and Transplant
Plasma taken from recovered patients contains antibodies that recognise the virus and can reduce its growth. We will be using plasma from patients at least 28 days after recovery as by that time, antibody levels will have increased.
NHS Blood and Transplant will contact people in England who have recovered from confirmed COVID-19 infection and could be a possible plasma donor, and the plasma will be collected at their centres. Blood will be taken from donors from one arm, which is circulated through a machine that separates out the plasma, and returned into the other arm. The process takes about 45 minutes and provides 2 units of plasma per donation, which can also be frozen and stored ahead for any future need.
Convalescent plasma was used as an effective treatment during the SARS outbreak.
If people have a confirmed positive test result and they are willing to donate, they can also provide details to us through NHSBT’s website.
You Make Also Like
England Increases Covid-19 Testing To 100K People A Day








